Certa Dose Receives Innovative Technology Contract from Vizient
- katpiercecloutier
- Aug 20, 2021
- 3 min read
Contract awarded for products that bring improvement to health care industry
Denver, CO. 8/1/21 Certa Dose announced its EPI for Anaphylaxis Kit and PALS Syringe Holder Kit has received an Innovative Technology contract from Vizient, Inc., the nation’s largest member-driven health care performance improvement company. The contract was awarded based on the recommendation of the EPI for Anaphylaxis Kit and PALS Syringe Holder Kit by hospital experts who serve on one of Vizient’s member-led councils, and it signifies to Vizient members unique qualities that potentially bring improvement to the health care industry.
Innovative Technology contracts are recommended after review and interaction with products submitted through Vizient’s Innovative Technology Program. Vizient member-led councils identify technologies that have the potential to enhance clinical care, patient safety, health care worker safety or improve business operations of health care organizations.
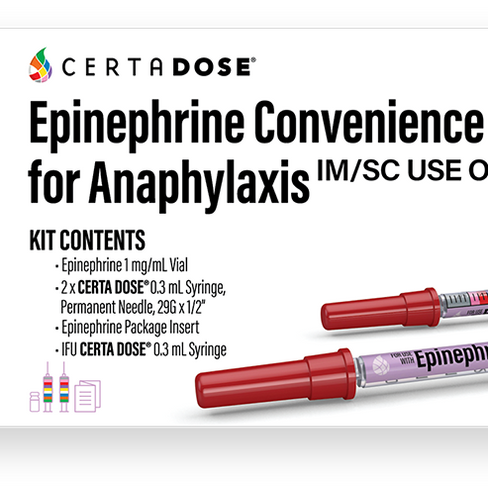
Certa Dose Epinephrine kit is an FDA-cleared system that helps offset the high cost of Epinephrine autoinjectors to treat allergic reactions that may arise due to Medication Reactions, Food Reactions, or Insect Bites and Stings. The CDC also requires Epinephrine for any Vaccination program, including the COVID-19 vaccination effort.
Healthcare systems that switch from Autoinjectors can realize ROI in a single quarter due to a lower unit cost, longer shelf-life, and ability of each kit to treat a wide range of patient sizes. Each device has easy-to-use dosing zones to help providers confirm the correct dose of Epinephrine for any size patient (from neonate to full-size). In addition, it’s the only system with a built-in double-check to confirm each patient receives the correct amount of this essential, life-saving medication regardless of their size, making it ideal for healthcare systems where patients of various ages are treated.
Pediatric “Code Blue” Arrest is one of the most challenging scenarios a healthcare team will face. These situations are high-stress, emotionally demanding, and wrought with errors, distractions, and mistakes. The Certa Dose PALS Syringe Holder Kit assists providers with a visual aid to quickly double-check and confirm the correct dose of these life-saving medications at the point of care. Each Syringe Holder has easy-to-recognize color zones that intuitively correspond to the classic Broselow colors for each pediatric age group. The Certa Dose PALS Kit assists healthcare providers when administering medication to children of various sizes without the need for a 3-way stopcock, saving valuable time and allowing providers to focus on the patient instead of the distractions so they can make a difference when every second counts.
“At Certa Dose, our mission is to enable healthcare professionals to navigate emergency dosing with certainty and deliver peace of mind. We aim to empower health care teams to be heroes when lives are depending on them. Making dosing mistakes a thing of the past and assisting providers to simply do no harm.” -Dr. Caleb Hernandez, CEO of Certa Dose
“A product receives this type of contract when it demonstrates a unique quality that differentiates it from other products on the market,” said Debbie Archer, director of procurement and Vizient Innovative Technology Program leader. “Our member council determined that Certa Dose’s EPI for Anaphylaxis Kit and PALS Syring Holder Kit met this standard and recognizes its potential to improve quality outcomes.”
Vizient represents a diverse membership base that includes academic medical centers, pediatric facilities, community hospitals, integrated health delivery networks and non-acute health care providers and represents more than $100 billion in annual purchasing volume. Through its Innovative Technology Program, Vizient works with member-led councils and task forces to evaluate products for their potential to bring real innovation to health care. Vizient may award a contract to products deemed worthy of the Innovative Technology designation outside of the competitive bid cycle.






Beyond the Beach: Exploring Hialeah Elite Female Escort Scene
Hialeah, a vibrant city located in Miami-Dade County, is known for its beautiful beaches, lively nightlife, and diverse culture. However, there is a hidden side to this city that is often overlooked - its elite female escort scene. While many may associate Hialeah with beach vacations and family-friendly activities, there is a thriving community of high-class escorts catering to the needs of discerning clients.
When it comes to finding female escorts near Hialeah, Harlothub is the go-to platform for both clients and escorts alike. With a wide range of services and a reputation for discretion and professionalism, Harlothub has become the premier choice for those looking for a memorable and luxurious…